
By Aliyah Kovner
Bio-based plastics such as polylactic acid (PLA) were invented to help solve the plastic waste crisis, but they often end up making waste management more challenging. Because these materials look and feel so similar to conventional, petroleum-based plastics, many products end up not in composters, where they break down as designed, but instead get added to the recycling stream by well-intentioned consumers. There, the products get shredded and melted down with the recyclable plastics, bringing down the quality of the mixture and making it harder to manufacture functional products out of recycled plastic resin. The only solution, currently, is to try to separate the different plastics at recycling facilities. Yet even with the most high-end, automated sorting tools, some bio-based plastics end up contaminating the sorted streams.
Scientists at Lawrence Berkeley National Laboratory (Berkeley Lab) and the Joint BioEnergy Institute (JBEI) are collaborating with X – the moonshot incubator led by Alphabet, Google’s parent company – to not only skip the problematic separation step, but also make the final product better for the planet.
The team has invented a simple “one pot” process to break down mixtures of petroleum-based and bio-based plastics using naturally derived salt solutions paired with specialized microbes. In a single vat, the salts act as a catalyst to break the materials down from polymers, large structures of repeating molecules bonded together, into the individual molecules called monomers, which the microbes then ferment into a new type of biodegradable polymer that can be made into fresh commodity products. The process is described in a One Earth paper published today.
“It’s sort of ironic because the purpose of using bio-based plastics is to be more sustainable, but it’s causing problems,” said first author Chang Dou, a senior scientific engineering associate at the Advanced Biofuels and Bioproducts Process Development Unit (ABPDU) at Berkeley Lab. Dou was recently named as one of the American Institute of Chemical Engineer’s 35 Under 35. “Our project is trying to get around the separation issue and make it so you don’t have to worry about whether you mix your recycling bin. You can put all the plastic in one bucket.”
In addition to streamlining recycling, the team’s approach could enable bio-based manufacturing of other valuable products using the same bacteria that are happily munching on plastic monomers. Imagine a world where biofuels or even medicines could be made from plastic waste – of which there is about 8.3 billion tons sitting around in landfills.
“There is an open discussion on whether we can use waste plastics as a carbon source for biomanufacturing. It is a very advanced idea. But we proved that using waste plastics, we can feed microbes. With more genetic engineering tools, microbes might be able to grow on multiple types of plastics at the same time. We foresee the potential to continue this study where we can replace the sugars, traditional carbon sources for microbes, with the processed hard-to-recycle mixed plastics that can be converted to valuable products through fermentation,” said Zilong Wang, a UC Berkeley postdoctoral researcher working at JBEI.
The Berkeley Lab scientists’ next step is to experiment with other organic salt catalysts to try to find one that is both highly effective at breaking polymers down and can be reused in multiple batches to lower costs. They are also modeling how the process would work at the large scales of real-world recycling facilities.
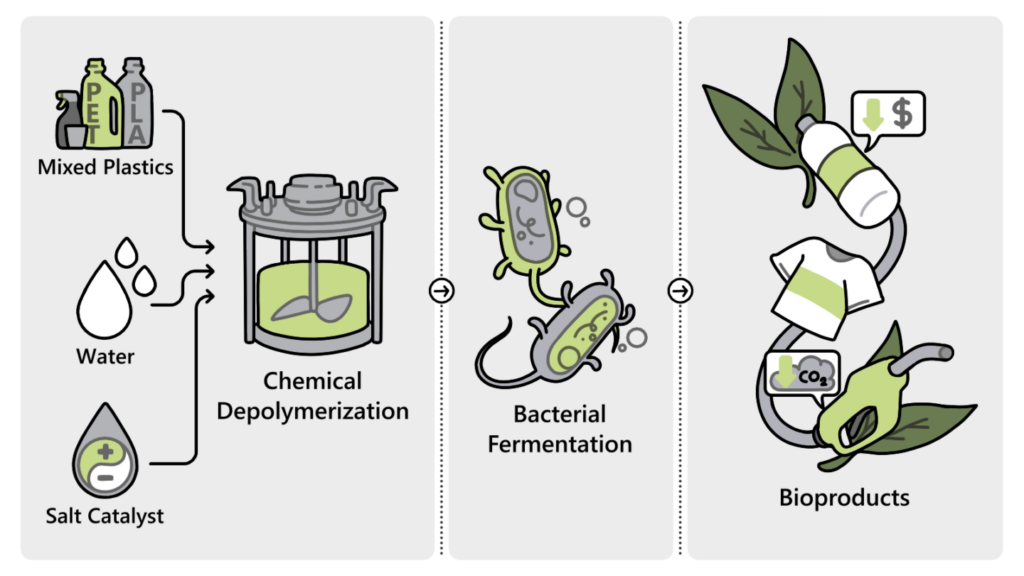
In their recent paper, the scientists demonstrated the potential of their approach in laboratory bench-scale experiments with mixtures of polyethylene terephthalate (PET) – the most common petroleum-based plastic, used in things like water bottles and spun into polyester fibers – and PLA, the most common bio-based plastic.
They used an amino-acid-based salt catalyst previously developed by colleagues at JBEI and a strain of Pseudomonas putida engineered by scientists at Oak Ridge National Laboratory. This combination successfully broke down 95% of the PET/PLA mixture and converted the molecules into a type of polyhydroxyalkanoate (PHA) polymer. PHAs are a new class of biodegradable plastic substitutes designed to efficiently break down in a variety of natural environments, unlike petroleum-based plastics.
Team member Hemant Choudhary noted that although their chemical recycling process is currently only proven for PET plastics contaminated with biodegradable PLA, it would still be beneficial for the diverse plastic streams encountered in real recycling facilities. “It can be completely integrated with existing plastic sources,” said Choudhary, a Sandia National Laboratories staff scientist working at JBEI. Most commercial products are not just one kind of plastic, but a handful of different kinds combined, he explained. For example, a fleece jacket is made with PET-based polyesters alongside polyolefins or polyamides. “We can throw it in our one-pot process and easily process the polyester component from that mixture and convert it into a bioplastic. These monomers are soluble in water, but the leftover parts, the polyolefins or polyamides, are not.” The leftovers can be easily removed by simple filtration and then sent off for a traditional mechanical recycling process where the material is shredded and melted, said Choudhary.
“Chemical recycling has been a hot topic, but it’s difficult to make it happen at the commercial scale because all the separation steps are so expensive,” said Ning Sun, a staff scientist at the ABPDU, lead author, and principal investigator of this project. “But by using a biocompatible catalyst in water, the microbes can directly convert the depolymerized plastics without extra separation steps. These results are very exciting, although we acknowledge that a number of improvements are still needed to realize the economic viability of the developed process.”
Co-authors Nawa R. Baral and Corinne Scown, experts in technoeconomic analysis in JBEI and Berkeley Lab’s Biosciences Area, also demonstrated that once optimized with a reusable salt solution, the process could reduce the cost and carbon footprint of PHAs by 62% and 29%, respectively, compared with today’s commercial PHA production.
JBEI is a Department of Energy (DOE) Bioenergy Research Center managed by Berkeley Lab. The ABPDU is a collaboration facility supported by the DOE BioEnergy Technologies Office.
This story originally appeared on the Berkeley Lab News Center.